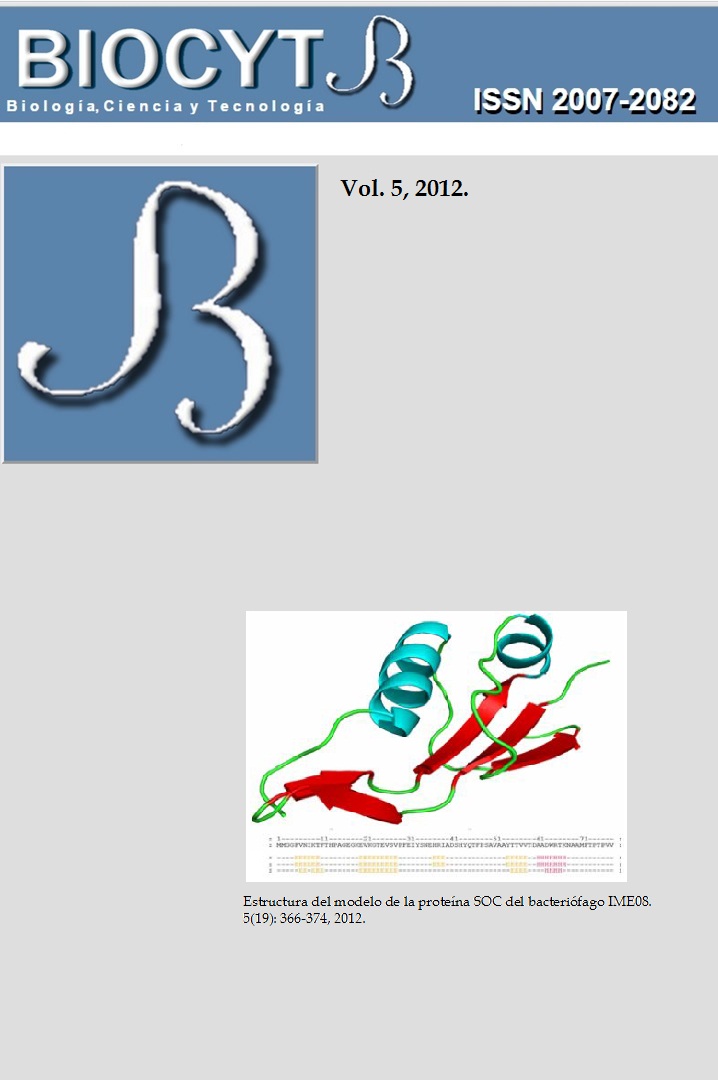
MODELACIÓN POR COMPARACIÓN Y ACOPLAMIENTO MULTIMÉRICO DE LA PROTEÍNA EXTERIOR PEQUEÑA DE LA CÁPSIDE DEL BACTERIÓFAGO IME08
Contenido principal del artículo
Resumen
The small outer capsid protein plays a stabilizing role in the viral assembly, adhering to the
capsid during the later stages of maturation. This protein acts as glue among adjacent
capsomers, protecting the virus against extreme changes. The small outer capsid protein of the
bacteriophage IME08 was modelled using structural protein homology. A trimeric protein
docking was developed with the best-scored model and important sites of the molecules
interfaces were identified. It was used the Swiss Model platform for developing the protein
structure. Reliability was assessed by the QMEAN, Verify3D and ERRAT indices. The quality of
the whole model was verified by Ramachandran plot and the trimerization model was
performed on the platform ClusPro 2.0 Protein-Protein Docking. The structure obtained has a
reliability estimator QMEANscore4 of 0.769, rating it as a suitable model. The Z-Score QMEAN
value was 0.133, showing that the obtained model is not different from the experimental
structures stored in PDB database. The estimators and the Ramachandran plot evaluated
positively the model. Finally we identified a loop between two secondary structures as an
important site of the interaction of small outer capsid proteins, indicating that from residues 35
to 41 are relevant in the trimerization process.
capsid during the later stages of maturation. This protein acts as glue among adjacent
capsomers, protecting the virus against extreme changes. The small outer capsid protein of the
bacteriophage IME08 was modelled using structural protein homology. A trimeric protein
docking was developed with the best-scored model and important sites of the molecules
interfaces were identified. It was used the Swiss Model platform for developing the protein
structure. Reliability was assessed by the QMEAN, Verify3D and ERRAT indices. The quality of
the whole model was verified by Ramachandran plot and the trimerization model was
performed on the platform ClusPro 2.0 Protein-Protein Docking. The structure obtained has a
reliability estimator QMEANscore4 of 0.769, rating it as a suitable model. The Z-Score QMEAN
value was 0.133, showing that the obtained model is not different from the experimental
structures stored in PDB database. The estimators and the Ramachandran plot evaluated
positively the model. Finally we identified a loop between two secondary structures as an
important site of the interaction of small outer capsid proteins, indicating that from residues 35
to 41 are relevant in the trimerization process.
Detalles del artículo
Cómo citar
Ospina-Bedolla, M. (2020). MODELACIÓN POR COMPARACIÓN Y ACOPLAMIENTO MULTIMÉRICO DE LA PROTEÍNA EXTERIOR PEQUEÑA DE LA CÁPSIDE DEL BACTERIÓFAGO IME08. BIOCYT Biología Ciencia Y Tecnología, 5. https://doi.org/10.22201/fesi.20072082.2012.5.76101